Abstract
Background: CPX-351 is a liposomal formulation of 5:1 molar combination of cytarabine and daunorubicin (Leukemia research 2010; 34: 1214). Subset analysis in a Phase II study reported improved outcomes in first relapse AML pts with European Prognostic Index defined poor-risk disease (Cancer 2015; 121: 234). In a phase 3 randomized study among fit pts, 60-75 years (yrs) of age with high-risk AML, that compared induction CPX-351 (100 U/m2, days 1, 3, 5) and vs 7+3; CPX-351 significantly improved response, event free and overall survival (Journal of Clinical Oncology 2016; 34(15_suppl): 7000). Observations from the Phase I study of responses occurring at dose levels of one third to one half the MTD (32 to 43 U/m2), suggest that doses of 75 and 50 units/m2 may still be effective and better tolerated among pts at high risk for toxicity.
Hypothesis: Reduced dose CPX-351 as induction therapy may improve the outcome of pts with AML at high risk for 60-day mortality and not ordinarily offered induction therapy.
Design: We designed an open-label, phase II trial of CPX-351 administered on days 1, 3, and 5 at 50 (arm 1) or 75 (arm 2) units/m2 as induction. Fifteen pts each were to be randomly assigned to these two arms. As these doses are below the original MTD dose of 100 units/m2, a cohort of 15 pts were to be studied at 100 units/m2 provided the first two dose levels were safe. At the end of the study a single dose level was to be chosen based on efficacy and safety i.e. dose limiting toxicity (DLT) <33%. Bayesian method was used for futility and toxicity monitoring. Observation of ≤3 CR/CRp/CRi among 15 pts (<20%) defines lack of efficacy. An expansion cohort of 10 pts were to be treated at the selected dose for evaluation for safety and efficacy.
The primary objective is to assess preliminary efficacy (CR/CRp/ CRi within 2 induction cycles) in pts with newly diagnosed AML at high risk for induction mortality, defined as 30-50% predicted risk of death by Day 60, and to select the most promising dose level for further efficacy testing. To be considered at high risk for induction mortality pts ≥60 yrs must have at least 1 and pts <60 yrs must have at least 2 risk factors that include antecedent hematological disorder, therapy related AML (T-AML), AML with MDS-related changes or MDS-associated karyotype, age ≥70 yrs, Performance Status ≥2, serum creatinine >1.3 g/dL. Pts with ejection fraction <50%, history of copper-metabolism disorder, uncontrolled infection or who received chemotherapy within 2 weeks of start were excluded. Responders could receive up to 4 consolidation courses of CPX-351 administered at 65 units/m2 on days 1 and 3.
Results: Based on safety of 2 dose levels (50 units/m2 and 75 units/m2) of CPX-351, one additional cohort with a dose of 100 units/m2 was opened. Fifty-two pts (15 at 50 U/m2, 25 at 75 U/m2 and 12 at 100 U/m2) have been treated (4 screen failures). Median age is 68 yrs (range 54-84) and 19 (37%) pts are females. Ninety percent of pts had prior MDS or T-AML, 81% had therapies for MDS and 54% had adverse cytogenetics. Patient characteristics are outlined in Table 1.
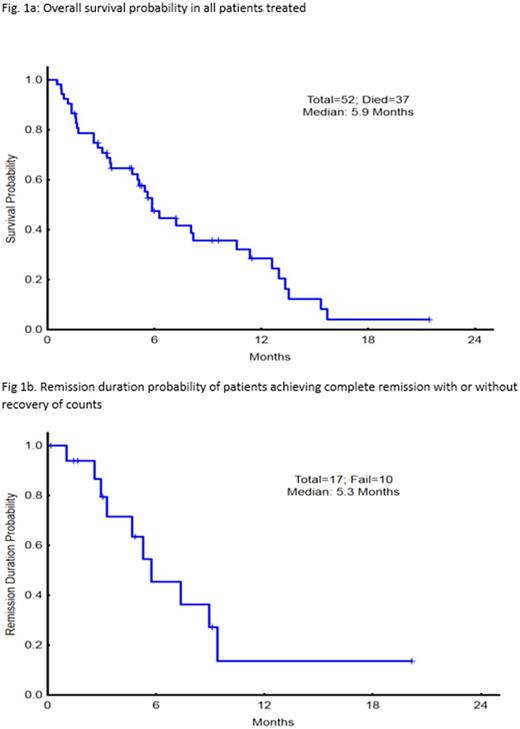
Median number of cycles to response has been 1 (range 1-2) and median number of cycles administered is 2 (range 1-5). Twenty (38%) pts have responded; 15 achieving CR, 3 CRi, 2 CRp. Six of 15 pts with CR were MRD negative at response. Responses (CR/CRp/CRi) are, arm 1=3/0/0 (20%), arm 2= 6/2/2 (40%) and arm 3= 6/0/1 (47%). With median follow up of 5.3 months, median survival in the entire cohort is 5.9 months and median remission (CR/CRp) duration 5.3 months (Fig 1a and b). Median time to recovery of counts for responding patients was 35 day (range, 16-153 days). Response rate trended to be lower among pts with mutant TP53 (20% vs 46%, p=.08), therapy related AML (0% versus 48%, p=.02) and younger than <65 yrs of age (21% vs 48%, p=.5).
Deaths within 60 days were 11 (21%), all due to sepsis, multi-organ failure or disease progression; 5 in arm 1 (33%), and 3 each in arms 2 (12%) and 3 (25%). Most frequent grade 3-5 toxicities, irrespective of attribution, were febrile neutropenia (38%) and respiratory failure (17%).
Conclusion : In a very high-risk elderly cohort of newly diagnosed pts with AML, CPX-351 induction yielded promising remission rates at all 3 doses tested. The efficacy, toxicity and early mortality profile favors the use of CPX-351 at 75u/m2 on days 1, 3 and 5 as the preferred induction schedule. Presence of TP53 mutation or T-AML potentially defines groups with low probabilities of response.
Kantarjian: Delta-Fly Pharma: Research Funding; Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Amgen: Research Funding. DiNardo: AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Daver: Bristol-Myers Squibb Company: Consultancy, Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Incyte Corporation: Honoraria, Research Funding; Kiromic: Research Funding; Immunogen: Research Funding; Karyopharm: Consultancy, Research Funding; Jazz: Consultancy; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Daiichi-Sankyo: Research Funding; Pfizer Inc.: Consultancy, Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Pemmaraju: LFB: Consultancy, Honoraria; stemline: Consultancy, Honoraria, Research Funding; affymetrix: Research Funding; cellectis: Research Funding; Incyte Corporation: Consultancy, Honoraria; novartis: Consultancy, Honoraria, Research Funding; roche diagnostics: Consultancy, Honoraria; abbvie: Research Funding. Wierda: Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Karyopharm: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Kite: Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Acerta: Research Funding; Juno: Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Merck: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding. Jain: Verastem: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Incyte: Research Funding. Cortes: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; ImmunoGen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal